
Unless the organisation is one person only, it is recommended that you have at least one other company administrator. You must carefully consider who is best placed in your organisation to be the initial company administrator as this person will be responsible for adding further company administrators and cascade responsibilities down through the organisation. It is recommended that each organisation has more than one company administrator.įor larger organisations, you may have different teams or departments who will be making submissions via MHRA Submissions, for example, regulatory affairs, pharmacovigilance units, clinical trials teams etc. There are different factors to consider when selecting this initial company administrator.įor smaller organisations, a company administrator may also be responsible for making submissions or managing a team who will make submissions.
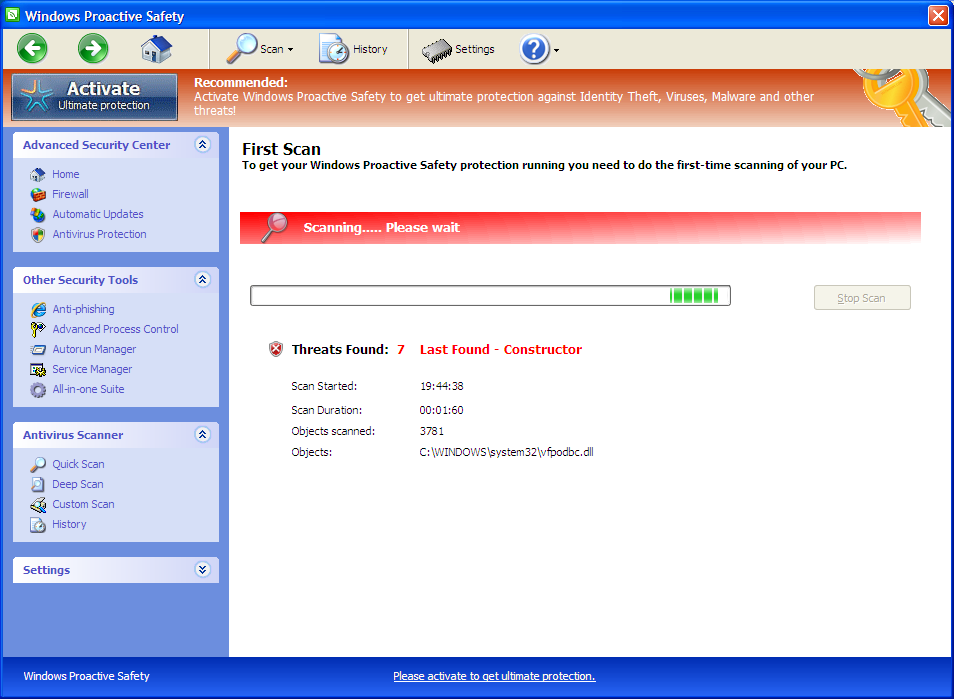
HOW TO REGISTER VFPODBC REGISTRATION
The first person in your organisation to complete the registration process outlined here will become the initial company administrator. User Reference Guide – Managing users on MHRA Submissions ( PDF, 540KB, 9 pages) The role of the initial company administrator User Reference Guide – Gaining access to MHRA Submissions ( PDF, 908KB, 20 pages) There are two user reference guides which contain step by step guidance on the processes:
HOW TO REGISTER VFPODBC HOW TO
Add a new external user – how to add a third-party consultant/consultancy as a user or company administrator.Add a new user – how to add an internal colleague as a user or company administrator.


You can access all three videos on Sharefile. Three short video demos are provided below which cover all aspects of the user access management process - these steps will enable your organisation to gain access and manage user permissions for using MHRA Submissions. Gaining Access to MHRA Submissions Before getting started - guidanceĮnsure that you have watched the videos and have referred to the user reference guides prior to clicking on the link in the Getting Started section. MHRA Submissions are used to send or receive ICSRs, the process for this can be found below. The steps for gaining MHRA Gateway access are contained within MHRA Submissions. Please note: all current EudraVigilance Gateway users who wish to gain access to the new MHRA Gateway will need to first gain access to MHRA Submissions.
HOW TO REGISTER VFPODBC UPDATE
all medicines clinical trial sponsors wishing to make clinical trial submissions (Initial Applications, Substantial Amendments, End of Trial Notifications and Developmental Safety Update Reports (DSURs)) to the Agency.all pharmaceutical companies involved in making medicines regulatory submissions and vigilance activities for UK/GB licences.The information on how to make submissions to the MHRA is for the following groups: For those regulatory submissions made through European procedures you will need to continue to submit via the EU portals (for example, CESP). For applications that you plan to submit to the UK (for example, a Marketing Authorisation for the UK or GB market), you will need to submit the information through our national portals.


 0 kommentar(er)
0 kommentar(er)
